SUMMARY
Shanghai Huaota Biopharmaceutical Co., Ltd. (hereinafter referred to as "Huaota") recently announced that the company's independently developed HB0045 project, the first CD73-targeting antibody cocktail HB0045, was administered into an ovarian cancer patient on October 3, 2023 at Gabrail Cancer Center, the top cancer research center in the United States. The patient is currently doing well and no adverse safety events have been reported.
Huaota will continue to rapidly advance the clinical Phase I dose escalation and dose expansion trials of HB0045, and actively explore its synergy by combining drugs targeting other molecules inside and outside the company’s pipeline to broaden the scope of indications. In this regard, Dr. Xiangyang Zhu, the general manager of Huaota, expressed his appreciation and expectations.
INTRODUCTION TO HB0045
CD73, also known as extracellular 5′-nucleotidase (NT5E), includes N-terminal and C-terminal domains. It exists as a homodimer on the cell surface and can also exist in free form outside the cell. As shown in Figure 1, the CD73 protein consists of 3 domains. The N-terminal domain (27-317AA) contains 2 zinc ion-binding sites and at least 1 N-glycosylation site located at aspartic acid at position 311; the C-terminal domain (337-549AA) contains the substrate binding site and is non-covalently bound to the plasma membrane through a GPI anchor; the N-terminal and C-terminal domains are connected by a short helix (318-336AA) , which controls enzyme movement. Therefore, CD73 exists in two conformations, "open" and "closed", and the catalytic reaction requires CD73 to convert from an open conformation to a closed conformation, which can expose the active binding site and allow substrate binding.
Figure 1. Illustration of CD73 conformation Changes
CD73 is expressed on various types of cells in the tumor microenvironment, including stromal cells, tumor cells, infiltrating immune cells, and epithelial cells. It is especially highly expressed in tumor cells and some infiltrating immune cells. In the tumor microenvironment, hypoxia induces the overexpression of CD73 on the surface of cancer cells, which dephosphorylates AMP to adenosine through CD73, forming an immunosuppressive tumor microenvironment and promoting tumor growth. Therefore, inhibition of CD73 restores T cell function.
HB0045 is a compound preparation composed of two monoclonal antibodies HB0038 and HB0039 targeting different epitopes of human CD73 in a molar ratio of 1:1. As shown in Figure 2 below, HB0038 can highly specifically bind to the catalytic domain composed of the N-terminus and C-terminus of CD73, forming steric hindrance to inhibit the hydrolysis of AMP (adenosine monophosphate) in a non-substrate competitive manner, thereby blocking Adenosine pathway. HB0039 can highly specifically bind to the N-terminal region of CD73, inhibiting the transition of CD73 from an inactive "open" conformation to a catalytically active "closed" conformation. It is an "allosteric inhibitor" of CD73, thereby inhibiting its enzymatic activity. Resulting in blockade of the adenosine pathway.
Figure 2. Binding modes of HB0038 and HB0039
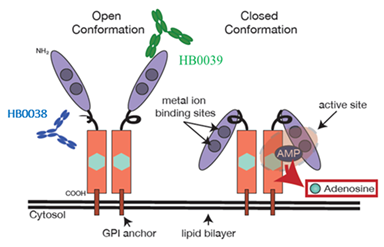
Figure 2. Binding modes of HB0038 and HB0039
HB0045 can completely inhibit the activity of membrane and soluble CD73 enzymes through dual mechanisms of allosteric action and steric hindrance without affecting the binding of AMP substrates, thereby reducing adenosine in the tumor microenvironment (TME). The product promotes the activation of TME immune cells and thereby inhibits tumor growth. In preclinical studies, compared with similar CD73 antibodies, HB0045 is the only one that can simultaneously target the catalytic region and N-terminus of CD73, and induce maximal inhibition of CD73 activity. It has shown better anti-tumor efficacy in preclinical animal models.
COMPETITION LANDSCAPE
At present, no drugs targeting CD73 have yet been launched. Oleclumab (anti-CD73 monoclonal antibody) developed by AstraZeneca has the fastest development progress and is currently in Phase III trials for the treatment of unresectable NSCLC after concurrent chemoradiotherapy. There are also many companies that are developing monoclonal antibodies against CD73 and are in early clinical and preclinical stages.