NEWS & MEDIA

The much-anticipated 32nd EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY (EADV) will be held in Berlin, Germany, from October 11 to 14, 2023. The EADV Congress is one of the largest international dermatology and venereology conferences, where experts in the dermatology field from all over the world will gather together to share the most cutting-edge research and progress in the field of dermatology.

Generalized pustular psoriasis (GPP) is a rare, life-threatening, systemic neutrophilic skin disease. The main symptoms of an attack are erythema and pustules all over the body, accompanied by systemic inflammatory symptoms. GPP has also been included in China's "Second Batch of Rare Disease Catalog".
At this meeting, Huaota will announce its positive data from a multi-center, open-label, single-arm exploratory Phase Ib clinical trial on the safety and efficacy of HB0034 in the treatment of acute exacerbations of moderate to severe generalized pustular psoriasis.
Title: novel anti-IL-36R antibody HB0034 shows positive efficacy in controlling acute GPP flare

About HB0034
HB0034 is an IgG1-type humanized monoclonal antibody targeting IL-36R independently developed by Huaota. It has high affinity for IL-36R, binds specifically to human IL-36R, and competitively blocks binding of agonists (IL36α, β and γ) to IL-36R. IL-36 is the key cytokine leading to inflammatory circuit, skin inflammation, pustule formation and abnormal tissue remodeling. Therefore, blocking IL-36 inflammatory pathway can reduce the release of inflammatory factors that drive disease to alleviate the disease. Numerous studies have shown that the IL-36 signaling pathway is closely related to the pathogenesis of GPP.
Research Background:
Generalized pustular psoriasis (GPP) is a rare, life-threatening skin disease with huge unmet clinical need. Abnormalities of the IL-36/neutrophil axis are closely related to the pathogenesis of GPP.
Research Methods/Results
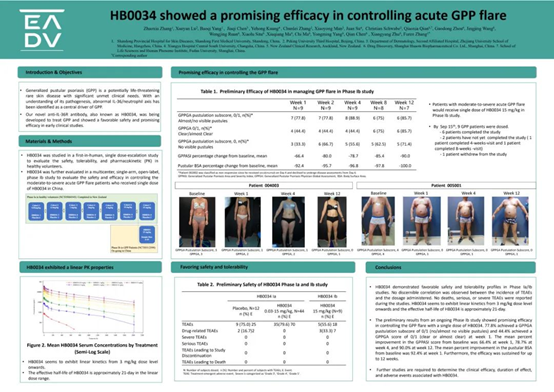
Phase Ia ascending dose study with single dose (HB0034-01) was conducted in healthy subjects in New Zealand to evaluate the safety, tolerability, pharmacokinetics and immunogenicity of HB0034 in healthy subjects. A total of 56 subjects were included in the study: 44 (78.6%) subjects received HB0034, and 12 (21.4%) subjects received placebo.
HB0034 demonstrated good safety and tolerability. No increase in the incidence of TEAEs was observed with dose escalation. No deaths, severe TEAEs, or SAEs were reported. HB0034, starting from the 3 mg/kg dose level, exhibited linear kinetics with an effective half-life of approximately 21 days.
The phase Ib of HB0034 was conducted in GPP patients in China. Clinical results showed that a single administration quickly controlled the acute attack of GPP, and the efficacy lasted for at least 12 weeks. After a single dose of HB0034, 77.8% of patients achieved a GPPGA Pustule Subscore of 0/1 (no/almost no visible pustules) and 44.4% of patients achieved a GPPGA Score of 0/1 (clear or almost clear) at week 1. In addition, the average percentage of improvement from baseline in GPPASI score after a single HB0034 treatment was 66.4% at week 1, 78.7% at week 4, and 90.0% at week 12. At the same time, the average improvement percentage of pustule BSA from baseline in the first week after a single HB0034 treatment reached 92.4%.
ABOUT HUAOTA
Huaota is a R&D enterprise focusing on independent development of biological drugs. The research and development of new biological drugs covers the fields of tumors, autoimmune diseases, and fundus lesions. There are currently 11 projects in the clinical stage, including key projects: 1) based on the impressive findings reported above, the first domestic self-developed anti- IL-36R monoclonal antibody for pustular psoriasis (rare disease), which is about to enter the critical phase II; 2) a PD-L1/VEGF bispecific antibody that has been in clinical phase II with observed positive signals in endometrial cancer and renal cancer; 3) the first CD73-ADC with multiple anti-tumor mechanisms.
Huaota is actively looking for domestic and foreign partners to co-develop key asserts to timely realize their potentials.